[Update] Intersessional Consultations on the Pandemic Accord: Countries Wrestle On Thorny Issues, The Fight is on To Get Text In
Newsletter Edition #32 [Treaty Talks]
Hi,
Reporting on closed-door negotiations is like looking into a black box. Hardly straight forward to decipher what is going on inside.
In today’s edition, we have tried to understand the proceedings in these informal consultations that have occupied member states as they move forward in their deliberations for a pandemic accord. Diplomats are hard at work engaging at multiple levels and across different fora, and in different configurations.
We hope you find this wrap on these various moving parts useful.
We need more countries to speak with us to ensure balance, accuracy and nuance in our reporting. We need more of you to support us so we keep at covering the evolution of these historic negotiations.
If you find our work valuable, become a paying subscriber. Tracking global health policy-making in Geneva is tough and expensive. Help us in raising important questions, and in keeping an ear to the ground. Readers paying for our work helps us meet our costs.
Until later!
Best,
Priti
Feel free to write to us: patnaik.reporting@gmail.com or genevahealthfiles@protonmail.com; Follow us on Twitter: @filesgeneva
STORY OF THE WEEK
Intersessional Consultations on the Pandemic Accord: Countries Wrestle On Thorny Issues, The Fight is on To Get Text In
With days to spare for the next meeting of the Intergovernmental Negotiating Body (INB) scheduled for July 17th-21st, countries continue to get bogged down by a convoluted process as they discuss substantive proposals on the text of the proposed instrument. While there is a realistic recognition on the difficulties and the challenge at hand, countries are also aware that crunch time has begun.
In a series of intersessional consultations that have taken place over the last few days, countries have not negotiated on the text per se, but have made statements on select provisions towards a Pandemic Accord. It is not fully clear whether all countries support the Bureau’s Text as the basis for negotiation, although it has become the de facto document that they are discussing.
The dynamics in these discussions illustrate the importance of process in structuring these sensitive negotiations, diplomats in Geneva say. The intersessionals so far have focused on certain topics, as decided by the INB, including on research and development; access and benefits sharing; and financing.
In this story, we attempt to bring you up to speed on some keys process and substantive issues that have emerged in recent days. We also discuss developments with respect to the medical countermeasures platform that also features in talks around the UN General Assembly meeting in New York in September this year. Finally, we discuss briefly the emergence of the equity group and what it means for these negotiations.
(Do note that, in parallel, countries also continued their engagement on intersessional meetings in the context of the IHR amendments. We will treat IHR discussions separately in a future story.)

“GETTING TEXT IN”: THE CIRCULAR JOURNEY
Before we discuss substantive issues that were taken up by countries in recent days, we look into what appears to be a confusing process that underlies these informal consultations.
Recall that at the end of the Drafting Group meeting in June, countries largely welcomed the Bureau’s Text. This was under the assumption that they will continue to add text to what has been proposed by the Bureau. But a number of diplomats from a range of countries told us, it has been a struggle to get text into the Bureau’s proposal. (See ours: Countries Take a Stab At Difficult Provisions in Bureau's Text This Week: Pandemic Accord Discussions)
“We supported the Bureau’s text on the assumption that was already proposed - as captured in the compilation text - will also feature in our discussions. But that has not been the case,” a developing country diplomat told us. The 200+ page compilation text, reflects all proposals made by countries.
In recent days, it is understood that some developed countries were of the view that the compilation text is “messy”.
Diplomatic sources from developing countries pointed out that “difficult” issues on intellectual property related provisions and those related to access and benefits sharing among others, were farmed out to be addressed in informal consultations. “Our text on these issues were already submitted earlier in April and they have been reflected in the compilation text. So we do not understand why are having to struggle to submit our proposals again into the Bureau’s text,” the developing country diplomat added.
Some developing countries are beginning to question whether this kind of a convoluted process has been by design in order to frustrate their efforts to include their suggested language on tricky issues. “Is the idea to buy time and block these proposals till the end so that there is no more time left?” asked one diplomat from a developing country.
(At the June INB drafting group meeting, countries including Germany, for example, also drew attention to certain proposals from the EU that did not make it to the Bureau’s Text.)
Experts who are tracking and involved in these negotiations say it is not clear why countries did not discuss clearly the status of the Bureau’s Text as a potential basis for negotiation. “If countries had concerns on the Bureau’s Text, why did they not bring it up?” one expert asked.
The outcome is that the process is now circular, with member states vying to put text back in. Precious time has passed with countries not making sufficient headway in actual negotiations, observers note.
Some countries express caution about starting with “a weak negotiation text” to begin with, that could put pressure on countries simply to strengthen the text before even negotiations commence.
Others see the Bureau’s text as a mere tool for consultations.
While the process may appear to be inefficient, the difficulty is in the nature of such negotiations where countries are rethinking new ways to incentivize research and development, or sharing information on biological samples, for example.
The enormity of the task is staggering, given the breathtaking scope of the discussions for a pandemic accord as they stand now. With barely ten months to the May 2024 deadline, the clock is ticking fast against the complex, multilayered discussions that these negotiations are.
It may be incorrect to infer that the process has been slow given the sheer breadth of issues involved. To be sure many areas that could potentially be covered under a new pandemic accord are highly complex, not to mention political. It is increasingly becoming clear that the deadline that countries set for themselves is unrealistic, as questions on a possible extension get louder in private conversations in Geneva. Already, countries are expecting that they may eventually buy time, but not right now, sources indicated.
The approach for the coming days is not clear, given that the informal consultations have not transpired in the way it was expected to, in terms of bringing countries closer in their positions.
“The informal consultations are not really working,” a diplomat from a large country shared. The idea was to bridge the gaps between positions of countries outside of the large INB meetings. “No one moved an inch. It appears countries have crystallized their positions,” the person said.
Not everyone is of the same view. A diplomat from a developed country found the informal discussions to be useful in getting a better understanding of the positions of various countries. “These processes take time, it is what it is. We have to be patient,” the diplomat told us.
Sources said that co-facilitators (two countries for each set of topics, who led the informal discussions), are expected to send a report on the consultations back to the Bureau of the INB. A number of countries told us, they were happy with the role played by co-facilitators particularly on research and development discussions.
There is, however, general discontent with the INB Bureau. As we reported earlier, concerns around the neutrality of Bureau members continue to be raised. We spoke with some experts who have watched WHO discussions over the years. Not all agree that Bureau members should be strictly neutral – in theory they represent different regions and are seen as a clearing-house for proposals from countries within their regions. However, countries do expect the Bureau to work as a team collectively to herd countries into closer positions. But in the case of the INB, many point out, that there are disagreements within the Bureau reflecting the high-stakes political issues involved in these negotiations.
THE INTERSESSIONALS:
WHO member states gathered a couple of times in recent days to discuss specific issues on Research and Development; financing; access and benefit sharing mechanisms.
ON R&D
Steered by Mexico and Norway as co-facilitators, countries discussed provisions for research and development (Article 9 in the Bureau’s Text). A proposal was developed by the co-facilitators that built on existing language from the Bureau’s text. It is also understood that Malaysia suggested additional language for Article 9, including the suggestion for a global strategy and plan of action for pandemics, sources familiar with the proceedings told us. Some suggested text to include an expert committee to help prioritize R&D for diseases and related medical products. Further provisions on commitments around publicly-funded R&D has also been suggested. New suggestions were also added to include obligations for WHO to strengthen coordination of R&D work for pandemic risk and products through the Global Observatory on Health Research and Development.
ON ACCESS AND BENEFIT SHARING
Countries also discussed Article 12 on access and benefits sharing at an intersessional meeting. These consultations were reportedly led by Ethiopia and Australia. One delegate described the discussions as “abstract”.
As before, some developed countries cautioned against a transactional approach linking the sharing of information to the sharing of benefits. (See Squaring the PABS circle in our previous story).
Sources involved in the discussions also suggested that there is an effort by mostly developed countries to dilute what is understood as “benefits”. Although specific proposals have been made by a range of countries, both developing and developed ones, on what constitutes benefits.
“In general, there is reluctance on the part of many developed countries to impose any kind of obligation on the industry,” a diplomat from a large developing country said. Experts also point to the existing ways in which information is stored, shared and transacted upon with many of these systems located or run by developed countries.
During the intersessionals, some developed countries reportedly questioned whether matters of genetic information could be linked to sovereignty. It was pointed out that these matters “were settled” in forums such as the Convention on Biological Diversity.
It is understood that some developing countries wanted to engage with the industry on ABS issues. This proposal was reportedly pushed back by others, citing that it is member states that should work on these provisions. The industry has not been in favor of crafting new rules on sharing information and to link to the sharing of benefits.
While early days yet, the PIP Framework model of governance where WHO signs contracts with manufacturers is emerging as a preferred policy route – at least for developing countries.
THE COMING TOGETHER OF THE EQUITY GROUP
A grouping of 20-25 developing countries have emerged as a counter-bloc to developed countries. The Philippines, on behalf of the Group for Equity, had made a statement at a previous INB meeting in June. This group of countries are discussing the possibilities for greater coordination on some of the issues of importance to developing countries in the context of the negotiations towards a Pandemic Accord. It is important to note that some countries that identify themselves as a part of the Equity Group are also part of the Friends of the Treaty Group that first pushed for a Pandemic Accord in 2021. The Friends group also includes developed countries.
Diplomats say that it is still early days for the Equity Group to arrive at common positions, given that there is diversity on some of the issues among these countries including on matters such as One Health, and Common but Differentiated Responsibilities, for example.
Bilateral and regional interests will also determine the extent to which developing countries will stick together to form a coalition.
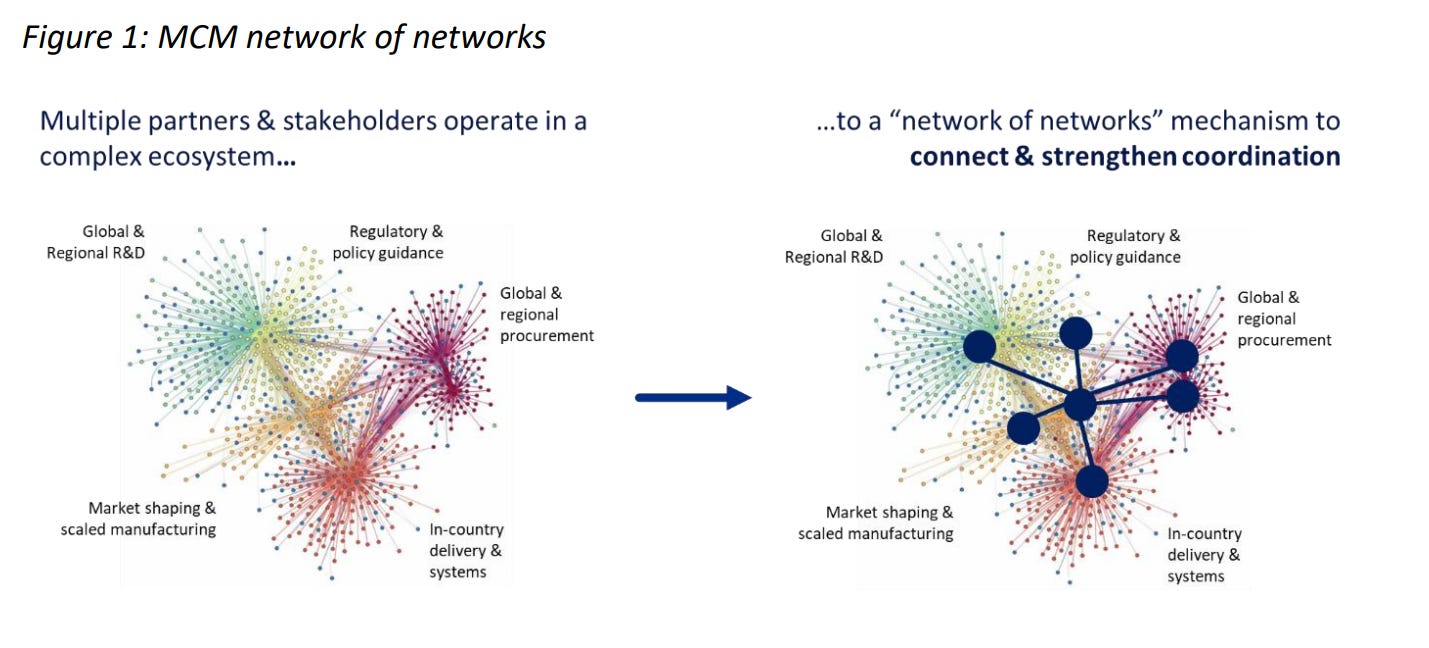
THE MEDICAL COUNTERMEASURES PLATFORM UP AND RUNNING BY SEPTEMBER?
WHO backed by key donors, is steering a new Medical Countermeasures Platform to ensure equitable access during health emergencies. In collaboration with a few countries, the private sector, foundations, and some global health agencies, WHO’s new proposed platform seeks to be “a multi-disease, multitool, end-to-end platform for coordinating the rapid development and equitable access to medical countermeasures for pandemics and major epidemics.”
While member states continue to discuss obligations around medical countermeasures in the context of the pandemic accord, and the amendments to the IHR, the discussions around this platform have taken up shape outside of these processes. As we reported recently, language on this platform has also surfaced in the UNGA-PPR text. (See: WHO Medical Countermeasures Platform Takes Route from New York to Geneva [UNGA-PPR Text]).
Recent documents, a draft concept note on the MCMs platform, seen by Geneva Health Files, indicate that the goal would be to get consensus from “WHO member states and other interested parties to facilitate discussion and consensus on the way forward ahead of the INB 6 (21 July) and United Nations General Assembly High-Level Meeting (UNGA HLM) on Pandemic Prevention (September 2023) and the conclusion of the India-led G20 and Japan-led G7 processes.”
Plans are on for launching the “MCM mechanism, including the secretariat, working group and, if agreed, an advisory group” in the run up to the September UN meeting. It is not clear why an interim mechanism has to be pulled together before even countries fully endorse or support such a mechanism.
The draft note does not refer to IHR discussions, but refers only to the negotiations at the INB. If the MCMs platform works outside of the obligations that countries have under the IHR, it could have implications on the nature of what such a mechanism sets out to do.
Apart from opposition from developing countries, and a few developed countries, the idea for such a platform has steadily gained support. Partners of the ACT-A, believe the “collaborative leadership” of a Public Private Partnership, such as the ACT-A, in order to deftly respond to emergencies in the future, while acknowledging the limitations of such a structure. The platform also seems to enjoy support from WHO leadership.
The scope of the mechanism has been identified as “Pathogens (or pathogen families) with pandemic potential as outlined in the R&D Blueprint for epidemics, including disease X and pandemic influenza; MCMs include but are not limited to diagnostics, therapeutics, medicines, vaccines, personal protective equipment, syringes and oxygen.”
The role is divided into categories of interpandemic and response period. The interpandemic period would include coordination activities, regulatory cooperation, identifying research priorities, establishing emergency supply chain networks and “promoting the geographical diversification of manufacturing and R&D capacities” among others.
The response period is envisioned with activities including prioritisation and facilitation of research efforts and mapping of candidate medical MCMs; determining the equitable allocation of available MCMs based on public health needs; facilitating the execution of advance purchase commitments, purchase agreements and pooled procurement for MCMs, drawing on and facilitating demand aggregation, among others.
Geneva-based sources also indicated that EU institutions are already looking to identify vendors for a MCMs platform. (Also see this from Reuters: EU secures vaccine deals with Pfizer, and others for future pandemic)
We leave you with this recent incisive analysis by Clare Wenham at the London School of Economics:
Creating more and more new institutions may not make the world safer from pandemics: “Given that amid WHO governance, there is one state one vote, there has been a recent move by richer countries to move this question of vaccine equity to a new multilateral platform for equitable access. This has notably established by those very governments that blocked the TRIPS waiver for intellectual property access for COVID19 vaccines during COVID, and can be seen to be moving the question of equity in terms of access to vaccines away from the auspices of a legally binding accord under which governments could be held to account for their action.”
Global health is everybody’s business. Help us probe the dynamics where science and politics interface with interests. Support investigative global health journalism.